Our Research Program
The Lo Laboratory is dedicated to advancing our understanding of cancer biology and developing innovative therapies for aggressive brain tumors and metastatic breast cancer.
Advancing Cancer Biology
Current Research Initiatives
Development of Novel tGLI1 Inhibitors for the Treatment of BCBM and Glioblastoma (GBM) Development of Novel tGLI1 Inhibitors for the Treatment of BCBM and Glioblastoma
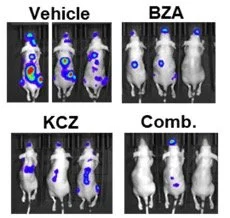
Given the tumor-specific expression of tGLI1, its high expression in BCBM and GBM sample, we hypothesized that pharmacological inhibitors of tGLI1 can effectively inhibit BCBM and GBM. Currently, there is no available tGLI1 inhibitors. Molecular structure for tGLI1 (or GLI1) is not available to allow for in silico identification of potential tGLI1 inhibitors. To initiate the effort in identifying tGLI1-targeted small molecules, we conducted cell-based screens followed by preclinical validations, and we identified an FDA-approved orally active antifungal KCZ to selectively target tGLI1-driven BCBM and effectively penetrate the BBB/BTB in mice without liver toxicities. These results have led to an active Phase 0 trial for BCBM and GBM (NCT03796273). We are continuing our efforts in developing effective and non-toxic tGLI1 inhibitors for the treatment of BCBM and GBM.
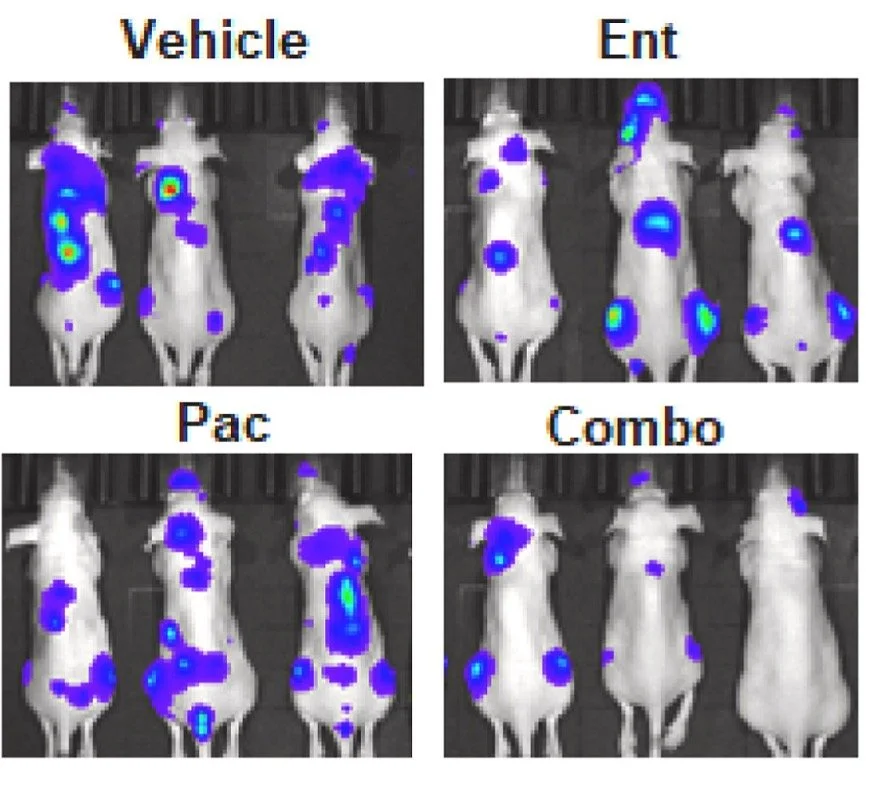
Co-targeting tGLI1 and IL-6R/GP130/STAT3 signaling
For the first time, we demonstrated that inhibiting these pathways synergistically suppresses HER2-enriched and triple-negative breast cancer (TNBC). This approach significantly reduced cancer cell proliferation and cancer stem cell phenotypes in vitro, while decreasing tumor growth and metastatic burden in vivo.
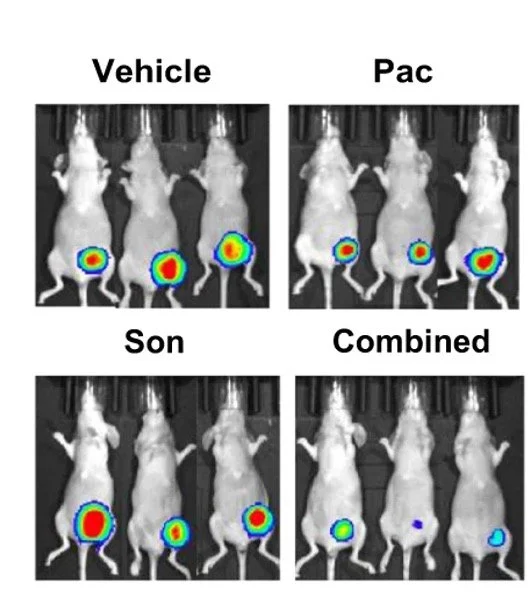
Dual inhibition of TrkA and JAK2/STAT3 pathways
Our studies revealed that targeting these pathways together synergistically suppresses breast cancer growth and metastasis. This preclinical evidence supports further clinical evaluations for HER2-enriched and TNBC subtypes.
Novel combinations of JAK2/STAT3 and SMO/GLI1/tGLI1 inhibitors
We determined that simultaneously targeting these pathways, which are co-activated and enriched in metastatic TNBC and HER2 breast cancers, has synergistic therapeutic effects in treating metastatic breast cancer.
Identifying Combination Therapies to Potentiate Cancer Treatment
The complexity and heterogeneity of cancer increase with advanced stages, making aggressive tumors more adept at developing resistance to single-agent therapies. As a result, such treatments often lose effectiveness over time or provide only temporary benefits. In our lab, we focus on identifying combination therapies that exploit cancer’s vulnerabilities to enhance treatment efficacy. Our approach leverages the biological evidence supporting cancer’s dependence on specific druggable targets. By co-targeting multiple oncogenic pathways, we aim to achieve synergistic effects that improve treatment responses, overcome drug resistance, and address the complexities of cancer progression. We are particularly focused on breast cancer metastases and glioblastoma, two malignancies that urgently require innovative therapeutic strategies. Through these and other ongoing efforts, our lab strives to develop innovative, multimodal treatments to improve outcomes for patients with aggressive and metastatic cancers
Recent breakthroughs from our lab include
Characterization of tGLI1 and miRNA-1290 for their roles in Breast Cancer Brain Metastasis (BCBM)
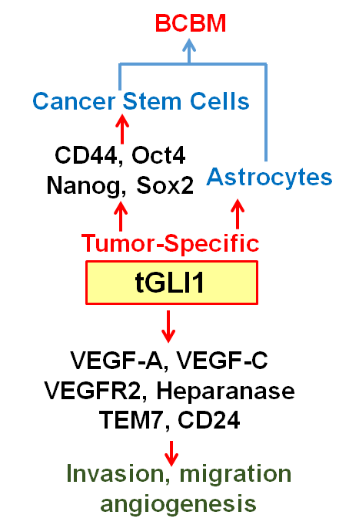
The goal of this project is to determine the role of tGLI1 (truncated glioma-associated oncogene homolog 1) in BCBM. Our laboratory discovered tGLI1 as an alternatively spliced GLI1 that lacks entire exon 3 and part of exon 4, but retains the ability to undergo nuclear transport and respond to sonic hedgehog-smoothened signaling. In addition to activating known GLI1 target genes, tGLI1 gains the ability to activate genes not regulated by GLI1, leading to increased migration, invasion and angiogenesis. Our mouse studies indicated that tGLI1 promotes breast cancer preferential metastasis to the brain (and conversely, tGLI1 knockdown selectively suppressed BCBM. tGLI1 protein is overexpressed in lymph node metastases and BCBM samples. In elucidating how tGLI1 promotes BCBM, we found that exosomes secreted from tGLI1-high cancer cells strongly activated astrocytes, the most abundant brain cells known to promote tumor growth when activated, and that tGLI1-high cancer cells had an increased ability to interact with and activate astrocytes in vitro and in vivo. Since microRNAs can be loaded into exosomes and circulating tumor exosomal miRNAs can prime distant organs for organ-specific metastasis, we conducted an exosomal miRNA microarray followed by validations and found that tGLI1-high cancer cells secreted high levels of exosomal miR-1290 and miR-1246 in vitro and in vivo. Whether miR-1290 and miR-1246 play any role in brain metastasis of any cancer or astrocytes is unknown. We observed that miR-1290/1246 inhibited expression of two transcription repressors (FOXA2 and SOX9), which leads to secretion of ciliary neurotropic factor (CNTF) to activate astrocytes and stimulate cancer cells. Ongoing projects in the Lo Lab are to identifying novel mechanism for BCBM.
Our Lab Mission
Unravel the complex signaling pathways driving cancer progression, with a focus on protein kinases, transcription factors, and non-coding microRNAs.
Investigate the molecular mechanisms underlying breast cancer brain metastasis and glioblastoma progression and drug resistance.
Develop and test novel targeted therapies and combination treatments for primary brain tumors and metastatic breast cancer.
Foster a collaborative, multidisciplinary research environment that bridges basic science and clinical applications.
Train the next generation of cancer researchers and clinician-scientists.
Through our integrated approach to cancer research, we aim to translate scientific discoveries into improved patient outcomes and contribute to the global effort in conquering cancer.